| Reaction |
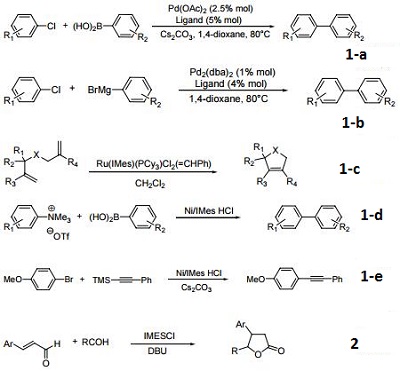
- Precursor to the nucleophilic carbene that serves as a bulky, electron-rich "phosphine mimic" for metal-catalyzed reactions.
(a) Palladium-catalyzed Suzuki cross-coupling of aryl chlorides.
(b) Palladium-catalyzed Kumada cross-coupling of aryl chlorides.
(c) Ruthenium-carbene catalysts for ring-closing metathesis.
(d) Suzuki coupling of aryltrimethylammonium salts.
(e) Sonogashira coupling of aryl bromides.
- Precursor to a nucleophilic carbene that serves as catalyst.
- Ligand for arylation of aldehydes.
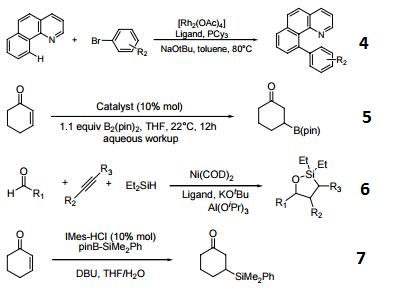
- Ligand for carbene catalyzed intermolecular arylation of C-H bonds.
- Catalyst for boron conjugate additions to cyclic and acyclic α,β-unsaturated carbonyls.
- Ligand for dehydrogenative cyclocondensation of aldehydes, alkynes, and dialkylsilanes.
- Precursor for carbene for conjugate silylation of alpha, beta-unsaturated carbonyls.

 |




