| Reaction |
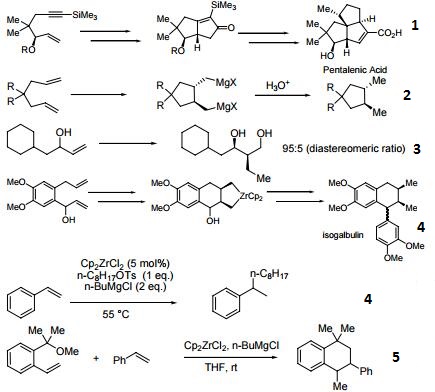
- Reagent for the conversion of enynes to bicyclic cyclopentenones.
- Precursor for the cyclization of dienes to cyclopentane and cyclohexane derivatives.
- Precatalyst for the alkylation of olefins.
- Precursor to zirconocene complexes of unsaturated organic molecules.
- Catalyst for the coupling of alkoxymethyl-substituted styrene derivatives.
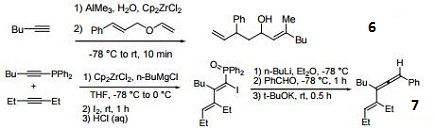
- Reagent for the carboalumination-Claisen rearrangement-carbonyl addition cascade reaction.
- Useful for the preparation of vinyl allenes.
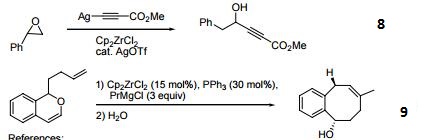
- Reagent for the alkynylation of epoxides.
- Catalyst for the formation of carbocycles from cyclic enol ether.
 |
|
|
| Chemical Properties |
White crystals. Soluble in
polar organic solvents. Stable in dry air, very slowly
hydrolyzes in moist air. |
|
|
| Uses |
Rubber accelerator, component of a catalyst
system for polymerization of vinyl monomers, curing
agent for water-repellent silicone materials,
agent for plating with zirconium. |
|
|
| Air & Water Reactions |
Bis(cyclopentadienyl)zirconium dichloride is extremely unstable when exposed to air. Decomposes in water . |
|
|
| Reactivity Profile |
Bis(cyclopentadienyl)zirconium dichloride is incompatible with water, acids, bases, alcohols and halogens. |
|
|
| Hazard |
Toxic by inhalation and skin contact, irritant
to eyes and mucous membranes. |
|
|
| Fire Hazard |
Flash point data for Bis(cyclopentadienyl)zirconium dichloride are not available, but Bis(cyclopentadienyl)zirconium dichloride is probably combustible. |
|
|
| Purification Methods |
Recrystallise the dichloride from CHCl3 or xylene and dry it in a vacuum. 1H NMR (CDCl3) : 6.52 from Me4Si. Store it dry in the dark under N2. [Reid et al. Aust J Chem 18 173 1965, Beilstein 16 IV 1770.] |
|
|




