| Reaction |
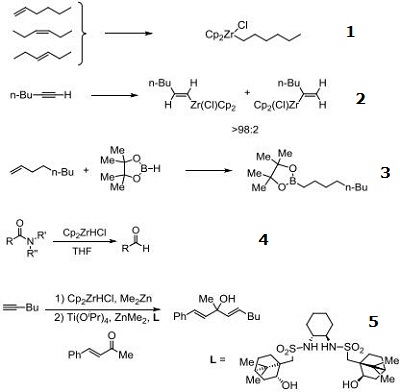
- Reagent for the hydrozirconation of olefins.
- Reagent for the hydrozirconation of alkynes.
- Catalyst for the hydroboration of olefins.
- Mediates the reduction of tertiary amides to aldehydes, without reduction of cyano, nitro, ester, or α,β-unsaturated groups.
- Catalyst for the asymmetric vinylation and dienylation of ketones.
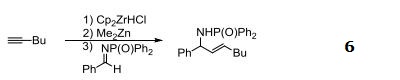
- Useful reagent for dimethylzinc mediated additions alkenylzirconocenes to aldimines.
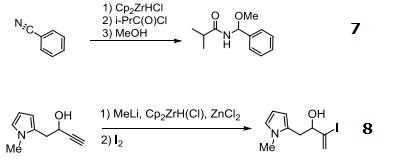
- Useful reagent for the synthesis of oxidized amides from nitriles.
- Reagent for the hydrozirconation of propargylic alcohols.
 |
|
|
| Chemical Properties |
white to beige powder |
|
|
| Uses |
Functionalization of unsaturated systems via an organozirconium intermediate. |
|
|
| Purification Methods |
It is moisture and light sensitive. Determine its purity by reaction with a slight excess of Me2CO whereby the active H reacts to produce Cp2ZrClOPri and the integrals of the residual Me2CO in the 1H NMR will show its purity. The presence of Cp2ZrH2 can be determined because it forms Cp2Zr(OPri)2. For a very active compound, it is best to prepare it freshly from the dichloride (see below) by reduction with Vitride [LiAl(OCH2CH2OH)2H2], the white precipitate is filtered off, washed with tetrahydrofuran, then Et2O and dried in a vacuum. Store it dry in the dark. [Carr & Schwartz J Am Chem Soc 101 3521 1979, Negishi & Takahashi Synthesis 1 1988, Beilstein 16 IV 1770.] |
|
|




