| General Description |
It is briefly referred to as TCA. It appears as colorless or white orthorhombic crystal with strong deliquescence. It has slight special irritating smell with strong corrosiveness. The aqueous solution of TCA is strongly acidic, and the aqueous solution of 0.1 mol of solution having a pH of 1.2. TCA with concentration of 30% or less is difficult to be stored for a long period of time since it is gradually decomposed into chloroform, hydrogen chloride, carbon dioxide, carbon monoxide and the like. In dilute alkali, it will be hydrolyzed into chloroform and carbon dioxide. Formic acid is formed in concentrated alkali. It is a strong corrosion products with a oral LD50 being 3320mg / kg.
Trichloroacetic acid is a strong organic acid with a dissociation constant K = 3 × 10-2. It has lively chemical properties. Its sodium salt is easily subject to decarboxylation into chloroform. It will be reduced to alcohol upon coming across LiAlH4. It can have halogen replacement reaction with KBr:
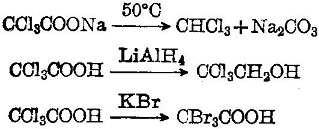 |
|
|
| Application |
Trichloroacetic acid can be used as pharmaceutical raw materials, herbicides (potassium trichloroacetate and sodium trichloroacetate, etc.), textile dyeing auxiliaries, metal surface treatment agent and acid chloride, anhydride, amide, polyester, organometallic salt, water salicylaldehyde, chlorocarboxylic acid and the raw materials of other organic synthesis.
In addition, in medicine, it can also be used as etherifying agents and keratolytics, bile pigment reagents and protein precipitation reagents. In the field of biochemistry, it can be used for separation analysis of biological phosphate compounds and reagents for determination of fluoride and lipid as well as microscopic fixative, decalcification, chromatography reagents.
The product is warts agent and astringent in pharmaceutical field, mainly used as biochemical drug extractant for the extraction of many highly efficient drugs such as adenosine triphosphate, cytochrome C and placental polysaccharides.
In addition, trichloroacetic acid, together with alkaline phenol, can be used for salicylaldehyde compound synthesis by ReimerTiemann reaction. It can also react with monoolefine compounds for synthesizing chlorocarboxylic acid [CCl3 (CH2CH2) nCOOH] . |
|
|
| Preparation |
- Use acetic acid or monochloroacetic acid as raw material; gradually warm in the 100~ 150℃ in the presence of the light or catalyst (ferric chloride) for chlorination to obtain it.
- Use hydrated chloral hydrate as raw material; apply oxidation reaction using concentrated nitric acid to obtain it.
|
|
|
| Uses |
Traditionally used to precipitate protein. Has been used to determine protein concentration by quantitative precipitation. Used as decalcifier and fixative, as a laboratory reagent, a herbicide, in medicine, and in microscopy. |
|
|
| Definition |
ChEBI: A monocarboxylic acid that is acetic acid in which all three methyl hydrogens are substituted by chlorine. |
|
|
| Chemical Properties |
Trichloroacetic acid, is a colorless crystalline solid. Trichloroacetic acid absorbs moisture from air and forms a syrup. Trichloroacetic acid is soluble in water with release of heat. Trichloroacetic acid is corrosive to metals and tissue. |
|
|
| Reactivity Profile |
Trichloroacetic acid is a strong acid; when heated, in the presence of water, decomposes forming phosgene and HCl. [Handling Chemicals Safely 1980 p. 915]. The acid was added to copper wool and rinsed down with dimethyl sulfoxide. This caused what was thought to be an extremely exothermic dehydrohalogenation reaction that melted the neck of the flask, [Chem. Eng. News, 1981, 59(28), 4]. |
|
|
| Health Hazard |
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. |
|
|
| Fire Hazard |
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form. |
|
|
| Purification Methods |
Purify the acid by fractional crystallisation from its melt, then crystallise it repeatedly from dry *benzene and store it over conc H2SO4 in a vacuum desiccator. It can also be crystallised from CHCl3 or cyclohexane, and dried over P2O5 or Mg(ClO4)2 in a vacuum desiccator. Trichloroacetic acid can be fractionally distilled under reduced pressure from MgSO4. Layne, Jaffé and Zimmer [J Am Chem Soc 85 435 1963] dried trichloroacetic acid in *benzene by distilling off the *benzene-water azeotrope, then crystallised the acid from the remaining *benzene solution. Manipulations should be carried out under N2. [Toxic vapours, use a well ventilated fume cupboard.] [Beilstein 2 IV 508.] |
|
|
| References |
https://pubchem.ncbi.nlm.nih.gov/compound/trichloroacetic_acid#section=Top
https://en.wikipedia.org/wiki/Trichloroacetic_acid |
|
|


