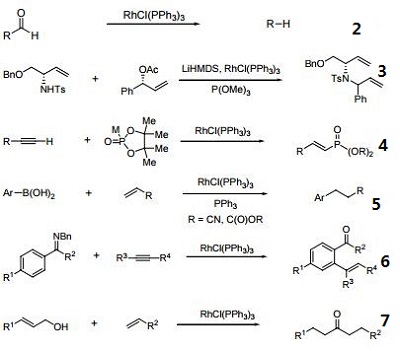
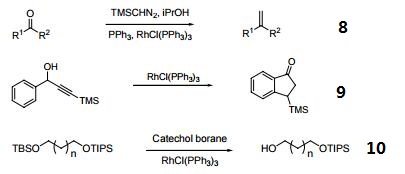
| Reactions |
- A homogeneous hydrogenation catalyst which operates under mild conditions.
- Catalyst for the decarbonylation of aldehydes.
- Catalyst for regio- and stereoselective allylic substitution reactions.
- Alkyne hydro-phosphorylation
- Heck-type reaction with α,β-unsaturated esters.
- Alkyne arylation
- Allylic alcohol-olefin coupling.
- Terminal alkenes from ketones.
- Rh-catalyzed isomerization of α-aryl propargyl alcohols to indanones.
- Reductive deprotection of silyl groups.
 |
|
|
| Chemical Properties |
magenta crystals |
|
|
| Uses |
suzuki reaction |
|
|
| Uses |
Tris(triphenylphosphine)rhodium Chloride is also known as Wilkinson's catalyst and is commonly used to catalyze the hydrogenation of alkenes. Tris(triphenylphosphine)rhodium(I) Chloride is also used i
n the catalytic hydroboration of alkenes with catecholborane and pinacolborane and the selective 1,4-reduction of α, β-unsaturated carbonyl compounds. |
|
|
| Uses |
Homogeneous hydrogenation catalyst. |
|
|
| Purification Methods |
It forms dark burgundy crystals from hot EtOH after refluxing for 30minutes. When the solution is heated for only 5minutes, orange crystals are formed. Heating the orange crystals in EtOH yields red crystals. Crystallisation from Me2CO gives the orange crystals. The two forms have similar IR spectra, but the X-ray diffraction patterns are slighly different. [Osborne et al. J Chem Soc (A) 1711 1966, Osborne & Wilkinson Inorg Synth X 67 1967, Bennett & Donaldson Inorg Chem 16 655 1977.] The solubilities are as follows: in CH2Cl2 ~2% (25o), in toluene 0.2% (25o), and less soluble in Me2CO, MeOH, BuOH and AcOH, but insoluble in pet ethers and cyclohexane. It reacts with donor solvents such as pyridine, DMSO and MeCN. |
|
|