| Reaction |
- (R)-BINAP or (R)-Tol-BINAP can be combined with dichloro(1,5-cyclooctadiene)ruthenium to form precursors to NOYORI CATALYST SYSTEMS. These systems exhibit very high catalytic activity and enantioselectivity in the hydrogenation of a wide range of substrates. NOYORI CATALYST SYSTEMS have been shown to effect highly enantioselective hydrogenation of functionalized ketones where the substituents are dialkylamino, hydroxy, siloxy, carbonyl, ester, amide or thioester.
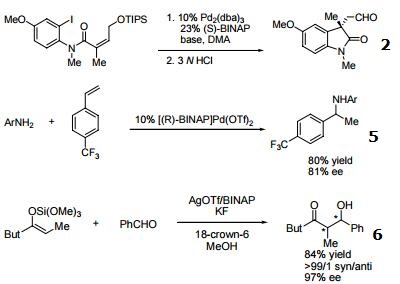
- Useful ligand in asymmetric Heck processes.
- Ligand employed in palladium-catalyzed asymmetric arylation of ketones.
- Ligand employed in rhodium-catalyzed 1,4-additions to enones.
- Ligand employed in palladium-catalyzed hydroamination of styrene derivatives.
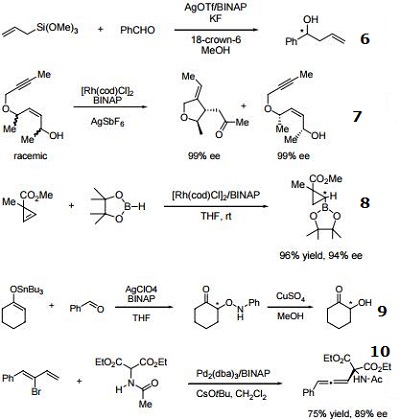
- Ligand employed in silver-catalyzed asymmetric Sakuri-Hosomi allylation and Mukaiyama aldol reaction.
- Ligand employed in rhodium-catalyzed kinetic resolution of enynes.
- Ligand employed in asymmetric rhodium-catalyzed hydroboration of cyclopropenes.
- Ligand employed in silver-catalyzed a-hydroxylation of stannyl enol ethers.
- Ligand employed in palladium-catalyzed synthesis of chiral allenes.
 |



